BIOVIA Databases
Easy Access to Integrated, Cross-referenced Data From Multiple Sources
Support Information-driven R&D
BIOVIA solutions support information-driven R&D by delivering unique answers to molecular property, chemical sourcing, bioactivity, and toxicology questions in the context of laboratory workflows, ultimately saving time, reducing costs, and enhancing decision confidence.
BIOVIA Databases are available in various formats that can be accessed via in-house installation or hosted service.
Deeply Integrated with the BIOVIA Portfolio
BIOVIA content is accessible out-of-the-box through:
Content to Support Your R&D
- Bioactivity
- Sourcing
Bioactivity
BIOVIA provides insight into druggability and intelligence on investigational drugs by providing access to one of the world’s leading collections of bioactivity data.
Toxicity
Anticipate adverse effects based upon chemical structure and link structure with metabolism information in the largest structure-searchable compendium of compounds with reported in vivo and in vitro toxic properties. Toxicity database includes Registry of Toxic Effects of Chemical Substances (RTECS®) database that is updated quarterly. Six categories of data types are covered:
- Acute Toxicity
- Mutagenicity
- Skin/Eye Irritation
- Tumorigenicity
- Reproductive Effects
- Other Multiple Doses
In addition, the following data sources (no further updates) are included in the Toxicity database.
- Chemical Carcinogenesis Research Information System (CCRIS), produced by the National Cancer Institute (NCI) ; Ref. 1938-2006
- Genetic Toxicity (GENE-TOX), produced by the U.S. Environmental Protection Agency (EPA) ; Ref. 1952-1987
- Genotoxicity, produced by genetic toxicity testing program of the US National Toxicology Program (NTP) ; Ref. 1983-1996
- Additional Carcinogenicity, Hepatotoxicity abstracted from scientific literature by MDL (now Dassault Systèmes BIOVIA) :
- BIOVIA Carcinogenicity; Ref. 2000
- BIOVIA Hepatotoxicity; Ref. 1999
RTECS
Registry of Toxic Effects of Chemical Substances (RTECS®) was built and maintained by the National Institute of Occupational Safety and Health (NIOSH) from 1971 through January 2001. Dassault Systèmes BIOVIA continues the production of the RTECS file using the same data selection criteria and rules established by NIOSH. BIOVIA distributes quarterly updates to licensees of the RTECS database under the terms of the License and Distribution Agreement entered into on October 29, 2001 between MDL (now Dassault Systèmes BIOVIA) and the United States Public Health Service within the Department of Health and Human Services. RTECS is a comprehensive collection of basic toxicity information for over 195,000 chemical substances from pharmaceuticals to foodstuffs.
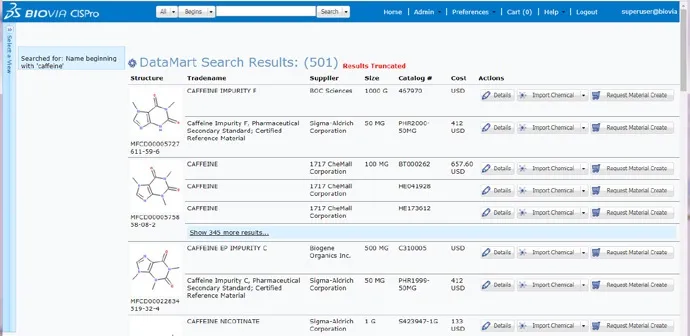
Sourcing
These structure-searchable databases provide supplier compound information including supplier, location, package, purity, and cost information helping you to make informed compound purchasing decisions and cut costs.
Available Chemicals Directory (ACD)
BIOVIA ACD is the Gold Standard for chemical sourcing in pharmaceutical, biotechnology, chemical, and agrochemical companies worldwide—used and trusted for more than 35 years by over 20,000 scientists at over 500 sites.
ACD consolidates chemical supplier catalogs into an easy-to-access, structure-searchable database providing information on:
- Product purities
- Available quantities
- Prices with supplier ordering information
- Forms
- Grades
- GHS classification for products
- SDS links to supplier product pages
BIOVIA ACD is one of the largest structure-searchable collections of commercially available chemicals in the world, with pricing and supplier information for:
- Over 15 million unique chemicals, including 2D structures and 3D models
- Over 45 million products
- Over 115 million packages
- From about 780 suppliers
With BIOVIA ACD you can:
- Identify and locate commercial sources
- Make side-by-side comparisons of purity, quantity and price
- Save valuable time by accessing information from a single source
- Identify the optimal set of compounds for a synthesis or library, with confidence that those compounds will be available at the indicated prices from the selected suppliers
Screening Compounds Directory (SCD)
BIOVIA SCD complements BIOVIA ACD by consolidating HTS supplier offerings into an easy-to-access, structure-searchable database of over 12 million compounds within commercially available sample collections.
Start Your Journey
The world of scientific informatics is changing. Discover how to stay a step ahead with BIOVIA.
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering