Regulatory Management
Reinventing Document Authoring with Structured Content
Achieve Data-Centricity, Digitalization and Automation in Regulatory Submissions
Regulatory Information Management is essential to ensure that sponsors can accurately and easily share content and collaborate with the relevant global regulators. Dassault Systemes is transforming submissions management by delivering a data driven and model-based approach to the automated generation of this critical content. Our ability to capture the essential data from the sponsors enterprise Data Lake or individual systems and generate the relevant CMC tables, charts and critical narrative is now possible. Initiatives such as KASA form the FDA mean that the industry must now leverage AI to transition away from conventional Word-based cut and paste from Lab systems.
Our CMC Authoring solution not only delivers the data, but also ensures full traceability to source, the ability to refresh the data dynamically, re-use of content between different regions/submissions, full “where-used” for all content elements, real-time collaboration across authoring groups and an unmatched ability to drive change control ad updates to CMC and other submission modules. This fully qualified Saas solution leverages state of the art AI and Structured Content Authoring techniques.
BIOVIA Regulatory Management Software Key Benefits
Compliance
Reduces errors caused by manual activities, ensures consistency and completeness of data and documents, ensure data traceability and “data integrity by design”, keeps up with agencies’ (FDA, EMA...) requirements including data format and reduces post-submission issues
Time and Budget
Reduces manual steps and non-value adding tasks, reduce manual data query of data in each application, eliminates repeated data verification approvals, reduce review cycles and reduces response time to authorities
Collaboration
Removes the complexity of multiple systems, collaborate simultaneously on documents, share knowledge based on structured content, formalize and harmonize internal practices and integrates new partners, suppliers and customers
A SaaS Cloud Solution
BIOVIA Regulatory Information Management solutions are available on the 3DEXPERIENCE platform to help deliver the benefits of a data-centric and AI-driven automated generation of documents for regulatory submission.
Key Benefits:
- Accelerate document production by eliminating manual transfer (copy and paste)
- Produce accurate reports through automated document generation
- Maximize transparency through traceability and direct access to source data
- Protect IP with a secure cloud-based collaborative environment
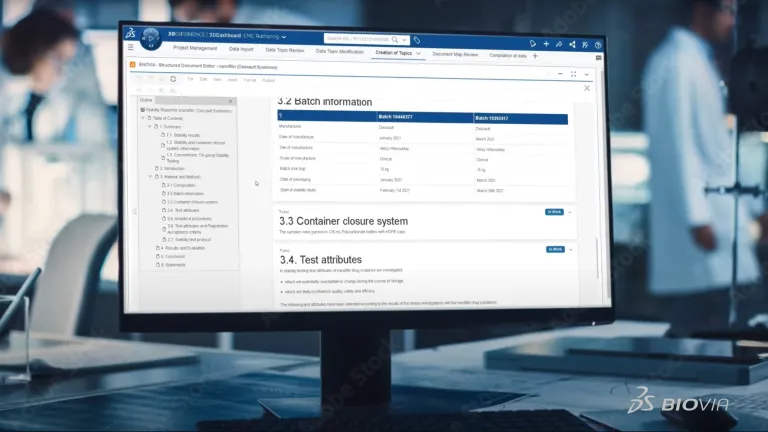
Transform CMC Dossier Creation
Streamline document creation by moving to a data-centric approach to CMC dossier creation. BIOVIA Structured Document Authoring reduces the time and cost associated with document creation through standardized formatting, automatic content updates, and secure collaboration.
FAQ About Regulatory Information Management System for Life Sciences
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering