QUMAS EDMS
Cloud-Based Electronic Document Management
Data-centric Approach to Document Management
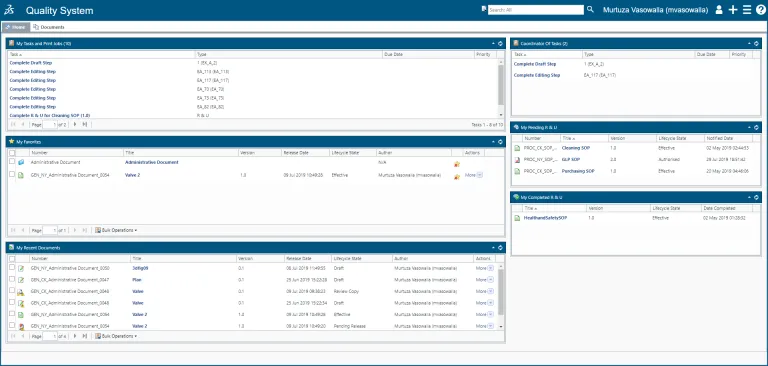
Documents and recorded data are the cornerstones of Quality and Compliance. However, organizations utilizing static documents cannot truly leverage quality data and adapt quality documentation to changing regulatory demands in an agile manner. BIOVIA QUMAS EDMS allows you to move from traditional document management to intelligent Quality Content Control. BIOVIA QUMAS EDMS is a cloud-based data-centric Electronic Document Management software solution that delivers proven regulatory compliance practices for data and document control, SOP management and related training across the business. QUMAS EDMS is an FDA 21 CFR Part 11 compliant document management system.
With BIOVIA QUMAS Document Management organizations can create and control policies, Standard Operating Procedures (SOPs), work instructions, manuals, files and reports dynamically, in compliance with global regulatory mandates. The ability to drag-and-drop content such as Word documents into the user interface simplifies document creation. The solution’s advanced data-centric approach facilitates document creation, search and management.
Customers Report:
-
20-40% cost reduction in SOP management
-
20-30 % time reduction in SOP review
-
30% cycle time reduction for documentation
-
60% increase in right-first-time submissions
Explore the Capabilities of BIOVIA QUMAS EDMS
- Enterprise Content Management
- Electronic Document Control
- Learning Management System - LMS
- Batch Data Management
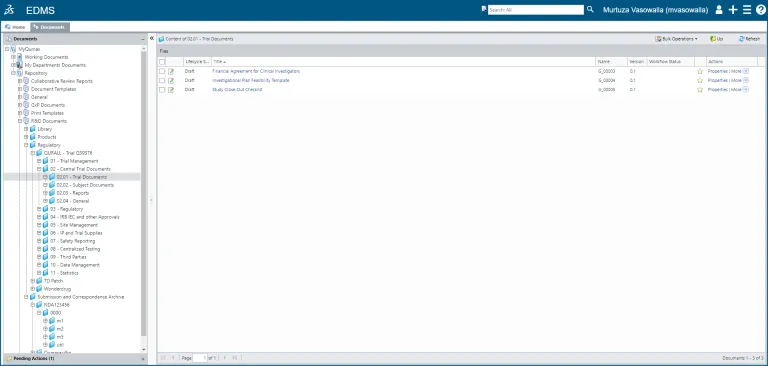
Control the full lifecycle of a document (or set of documents), from creation through content editing, review/approval and hardcopy management to scheduled retention and retirement.
Author and manage policies, Standard Operating Procedures (SOPs), work instructions and manuals - for electronic Common Technical Document (eCTD), non-eCTD and Regulatory Submissions, CMC (Drug Product and Drug Substance), clinical, non-clinical and Quality.
Automate the process for compliance training and management or launching, tracking and managing interactive corporate compliance training (powered by NetDimensions).
Handle large quantities of documents efficiently with dedicated capabilities:
- Enterprise Scanning
Automated scanning for converting legacy paper archives to electronic documents with metadata, creating cover pages or printing batches of forms to be reconciled and archived - Document Transfer (DocTransfer)
Automated batch load of multiple documents from different applications through metadata definition maintaining data integrity; drag-and-drop a set of files from the file system - Content Cache
Storage of a local cache of frequently used documents for widely dispersed global deployments and for better network performance
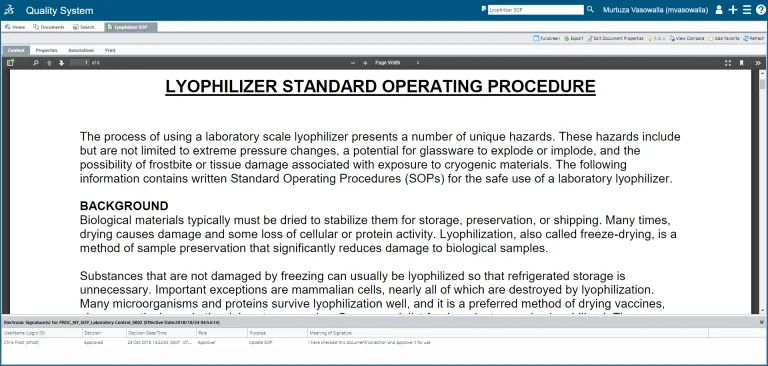
SOP Management
QUMAS EDMS automates the creation, sharing, distribution and management of SOPs across a global network and tracks SOP lifecycles.
- Compare versions for easy identification of updates.
- Access to the SOPs that are current and relevant to your work.
- Search for content, view it and print it according to permissions.
- Ensure that the version you are using is the current, controlled version of the document with a fully compliant audit trail.
- Be compliant with all cGxP requirements for procedure management as well as with 21 CFR Part 11.
eCTD & Submission Management
Preparing an electronic common technical document (eCTD) for a drug application is a complex and time-consuming process. Creating and approving responses in an environment with disconnected systems can delay time-to-market for new drugs and reduce the profit window before patents expire.
BIOVIA QUMAS combines in one integrated solution:
- Template-based content authoring and management
- Real-time collaboration and submission management for Regulatory Affairs, Clinical, Nonclinical and Quality
- Automated electronic submissions (including CTD, eCTD, NeeS, IMPD, CTA, eNTA and VNeeS formats) to regulatory agencies like the FDA, EMA or PMDA in a compliant manner
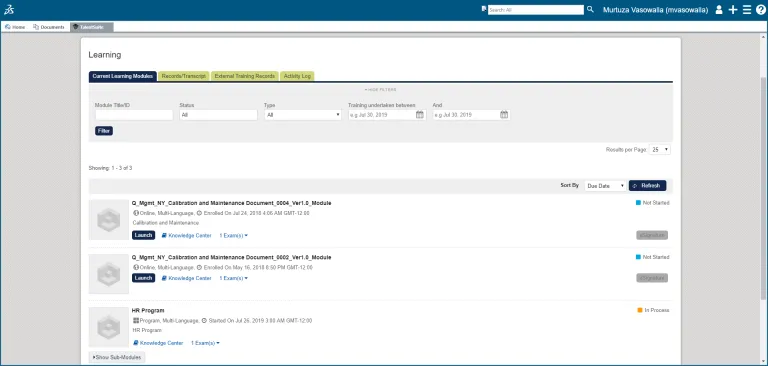
Learning Management
Employees can complete training on relevant SOPs and acknowledge their comprehension and understanding.
Each employee has a record of training tasks they have been assigned, any that are outstanding, and a complete history of which training tasks that have been completed.
Management reporting and dashboards provide you with a clear view of the status of individual and departmental training and certification, evidence and records to prove this to regulatory authorities.
Industry Process Accelerators - IPAs
The BIOVIA QUMAS EDMS Industry Process Accelerators include dedicated configurations, documentation (e.g., a validation pack, design document, system access plan), professional services (installation, end user and train-the-trainer training) and professional services review for at least 3 months after go-live. It includes configuration, documentation, validation scripts, and design documents.
- Quality Assurance Documents IPA
- R&D Submission Documents
Quality Assurance Documents IPA
Supports the lifecycle management of your Quality Assurance (QA) documentation. Configurations are specific for QA use and based on widely accepted business fundamentals. They include 5 pre-defined GxP document types, procedures, methods, specifications, regulatory and general, and 5 pre-defined workflows for content progression and management.
R&D Submission Documents IPA
Supports your submissions to global regulatory authorities. Configurations are specific for R&D and follow the DIA EDM reference model and CTD standards. They include 14 pre-defined document types like CMC - Drug Product, CMC - Drug Substance, Specifications, Clinical and Nonclinical.
Start Your Journey
The world of Biopharma Quality is changing. Discover how to stay a step ahead with BIOVIA.
Also Discover
Learn What BIOVIA Can Do for You
Speak with a BIOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right BIOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering