Oncology
Better understanding and treating cancers with MEDITWIN.
Better understanding of the disease
In 2018, cancer was the second leading cause of loss of healthy life years (disability-adjusted life years, DALYs) worldwide, responsible for approximately 1 in 6 deaths [1-2]. Between 2010 and 2019, the number of new cancer cases worldwide increased by 26.3% and the number of deaths by 20.9% [2].
Virtual twins experienced in oncology practices help increase the expertise of professional teams and individuals, notably by:
Combining information to predict the effect of interventions
Why characterize the stage of cancer progression?
When cancer is diagnosed, management depends on the extent of the disease in the body and its biological profile. This is known as staging.
It is essential to combine increasingly complex information from a series of tests, including biological analyses, imaging, and endoscopic examinations, coupled with biopsies.
The stage characterizes the development of the disease in the primary organ, in the lymph nodes, and in organs distant from the primary organ.
The effect of treatments depends on the stage (and the biological profile). This is why staging allows surgical or radiological procedures, radiotherapy, and drug treatments to be planned and adjusted on a case-by-case basis through multidisciplinary discussions.
Virtual twins would make it possible to apply this knowledge to each individual. The goal of the MEDITWIN project is therefore to create virtual representations of the disease to assist with staging and intervention planning.
What are the difficulties involved in managing the care of individuals with this disease?
- International standards for cancer staging are essential but complex to implement without a digital solution. A patient must undergo a large number of tests (endoscopy, MRI, CT scan, biopsies, etc.). These tests are becoming increasingly numerous and time-consuming to analyze, and they are not all reviewed by the same doctor. Understanding and taking into account all this multimodal and multi-scale data is a challenge for healthcare professionals and patients alike.
- Interventions must be personalized. This is the case with the heat-based destruction or thermal ablation of liver metastases, which requires personalization and a high level of clinical expertise. Taking into account numerous parameters to adjust the technique, including the size of the lesion, the proximity of large vessels, and the type of energy used to generate heat, requires a high level of expertise. A good understanding of the tumor and its growth rate is also required.
To concretely illustrate this personalized therapeutic pathway, we present below video excerpts corresponding to the different key stages of the procedure: planning, guidance, ablation, and post-procedure monitoring.
These videos come from real cases conducted on the GLP-certified (Good Laboratory Practice) preclinical experimentation platform at the Strasbourg University Hospital Institute (IHU), under the supervision of Dr. Juan Verde [3-4].
Video excerpts illustrating the key stages of the personalized therapeutic pathway
What do the virtual twins from the MEDITWIN project contribute?
The project's virtual twins are designed to help define the best therapeutic strategy for each individual. They enable the integration of multiple medical examination approaches and the execution of virtual scenarios. The studies will focus on treatments for people living with colon and rectal cancer, but the principles are applicable to almost all types of cancer.
Three Inria project teams are involved alongside Dassault Systèmes in the creation of these virtual twins:
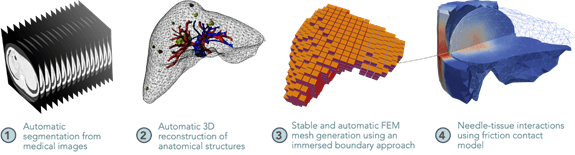
The MIMESIS project team will specialize in real-time multi-physics biomechanical simulations, using in particular the smoothed finite element method (SFEM) and data-driven simulation (medical image assimilation, multimodal 3D registration). These biomechanical models will enable dynamic and predictive visualization of oncological interventions on the liver (thermal ablation of liver metastases). The team will thus assist surgical decision-making by ensuring greater precision and safety during locoregional treatments.
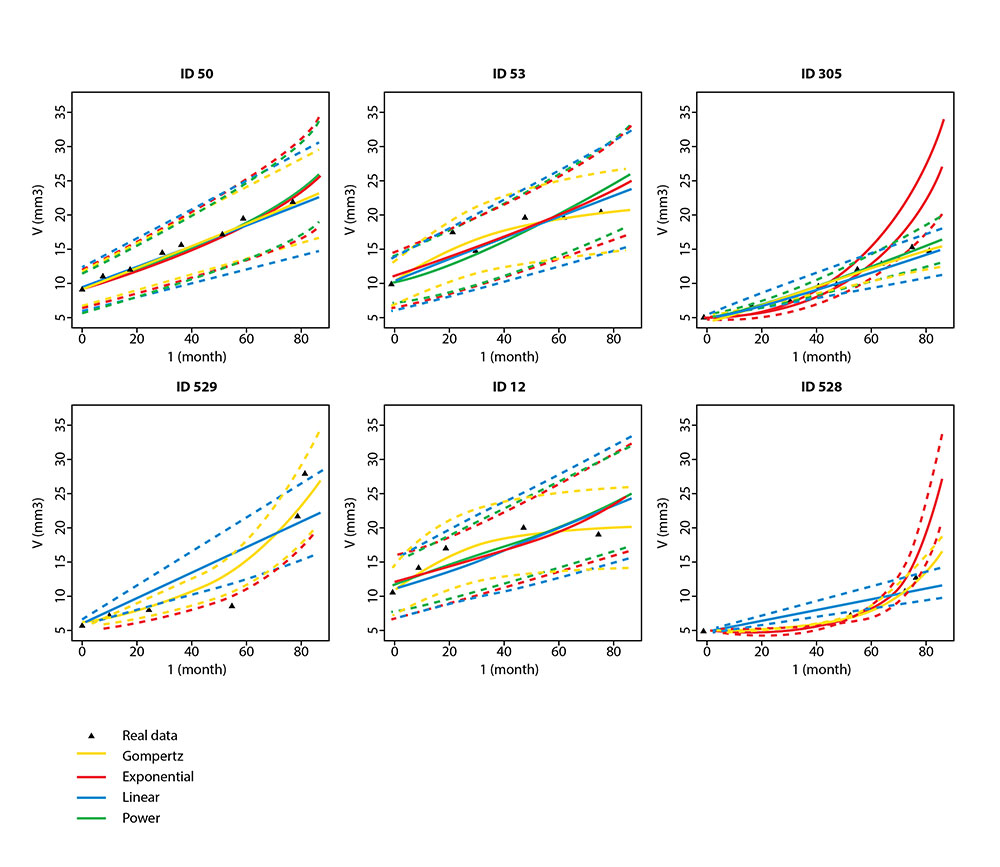
The MONC (Modeling in ONCology) project team will build mathematical models of tumor growth based on partial differential equations (PDEs) coupled with advanced statistical methods for data assimilation (medical imaging, biological and genetic data). These numerical simulations, calibrated using patient-specific data, will make it possible to anticipate tumor progression, the appearance of metastases, and individual therapeutic response, thereby improving clinical stratification and the personalization of cancer treatments.
The HEKA project team will provide a multidisciplinary decision support solution for the diagnosis, treatment, and monitoring of cancers based on a virtual twin reconstructed from whole-body or partial-body imaging, histopathology, biological, clinical, and genetic markers of patients, the tumor, and tumor organoids.
Developing precision medicine tools for cancer
What is precision medicine?
Cancer is a complex disease that evolves over time and space.
The current shift towards precision medicine recognizes the importance of the biology of each cancer. This new practice recommends studying the genetic, immune, and environmental information of the cancer to identify an appropriate treatment.
Thus, the treatments used are better targeted and tailored to each individual.
What will the precision medicine experience be like with MEDITWIN?
The MEDITWIN project aims to map the profile of each cancer and take into account all patient data (multi-scale, biological, psychological, social, etc.) for the selection of new therapies or participation in a clinical trial.
Patient information and care management are facilitated by the representation of medical knowledge combined with individual data to present patients with therapeutic options tailored to their situation, with levels of evidence established by the latest scientific knowledge.
Why a research project on organoids?
Organoids are in vitro cultures of tumor cells extracted from biopsies. They allow potential treatments to be tested in vitro before being administered to a patient. Organoids are a promising avenue of research for precision medicine.
While in vitro testing is slow and expensive, the creation of virtual organoids would make it possible to test a large number of potential treatments, select which ones to test on in vitro organoids, and give more patients access to organoids.
References