Neurology
Better understanding and treating neurological diseases with MEDITWIN.
Contextualizing the Disease
Neurological diseases are linked to complex dysfunctions of the nervous system that can be of genetic, developmental, or traumatic origin, but are also related to aging, which is responsible for the growing increase in neurological diseases in many countries.
In 2021, these diseases affected more than 3 billion people worldwide and were the leading cause of poor health and disability worldwide. The total number of disabilities, illnesses, and premature deaths caused by neurological diseases has increased by 18% since 1990 [1].
A better understanding of nervous system diseases is necessary to implement preventive measures and more targeted therapeutic management.
The MEDITWIN project will seek to:
Understanding the development of Alzheimer's disease and vascular dementia for early management
Context of the disease
Alzheimer's disease and vascular dementias are characterized by memory impairment, difficulty performing simple everyday tasks, problems with speech and writing, and disorientation in time and space. Alzheimer's disease is the most common neurodegenerative disease worldwide, affecting nearly 50 million people. In the coming years, this number is expected to double or even triple.
Alzheimer's disease and vascular dementias do not have the same causes or treatments, so it is crucial to be able to identify them. In both cases, the earlier the diagnosis, the sooner a multidisciplinary care strategy can be put in place, and the better the chances of delaying the onset of symptoms and preventing the deterioration of the patient's health through compensatory and brain plasticity mechanisms.
Medications aimed at slowing down or even "reversing" Alzheimer's disease are gradually becoming available. Their aim is to "clean up" lesions in the brain. Up to 80% of brain lesions can disappear with these treatments, but they are only effective if prescribed in the early stages of the disease.
Early diagnosis of Alzheimer's disease is becoming more important than ever, but remains a challenge for the medical community. Artificial intelligence and virtual twins will be able to help create simple, reliable, and non-invasive tests. One possible approach is to predict the results of invasive tests using artificial intelligence-based methods.
What are the challenges in managing the care of individuals living with Alzheimer’s disease?
There are currently two types of diagnosis: early diagnosis and definitive diagnosis.
- Early diagnosis is performed in the hippocampal region of the brain. This is where the lesions of the disease begin to appear.
- Definitive diagnosis confirms the disease by highlighting the lesions caused by the disease through PET scans and lumbar punctures. The PET scan is an advanced medical imaging examination used to visualize certain brain lesions characteristic of Alzheimer's disease, such as deposits of tau proteins, one of the neurotoxic proteins responsible for this disease. It is currently an essential tool for confirming a diagnosis. However, these examinations remain complex, costly, and not widely available.
How does the MEDITWIN project contribute to the early diagnosis of Alzheimer's disease?
Using virtual twins, new diagnostic tests will be developed and assessed the onset and to predict the progression of Alzheimer's disease based on retinal markers, EEG biomarkers, and PET scan images.
- Retinal markers
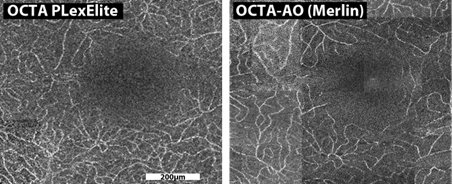
The retina, located at the back of the eye, originates from the same embryonic structure as the brain, which explains why both share a similar vascular network. The study of its blood vessels and cells can reveal early signs of neurodegenerative diseases and help identify Alzheimer's disease and vascular dementias.
This is made possible through adaptive optics, a unique imaging technology that corrects distortions from the eye’s structures to achieve cellular-level resolution. Developed for astronomy and first applied to vision research in Europe by the Institut de la Vision and the 15-20 National Hospital in 2005, adaptive optics enables visualization of photoreceptors, ganglion cells, and microvascular structures. By identifying retinal biomarkers at this scale, it opens the way to automated detection, precise monitoring of diseases, and real-time evaluation of treatments.
- EEG biomarkers
Electroencephalography (EEG) is a non-invasive method that records the brain’s electrical activity through the scalp in real time. It is used to measure neural oscillations, their synchronization, and their responses to sensory or cognitive stimuli. In the context of Alzheimer's disease, EEG can reveal early alterations in brain function, sometimes before the onset of clinical symptoms. These electrical signatures can thus become dynamic biomarkers of brain aging, potentially useful for screening and personalized monitoring.
To validate these biomarkers, it will therefore be essential to standardize real-life EEG recordings and demonstrate their ability to predict long-term cognitive decline.
- The use of PET images
PET scans can detect lesions characteristic of Alzheimer's disease, but they remain difficult to access for a large number of patients. To overcome this limitation, the MEDITWIN project is developing an artificial intelligence algorithm capable of estimating the probability of a positive PET scan result based on data that are easier to collect, such as memory tests or brain imaging examinations.
Combined with other neurological tests, this tool will be directly integrated into the hospital neurologist’s working environment. It will thus help them detect the signs of Alzheimer's disease more systematically and at an earlier stage, and better guide patient follow-up.

A positive test result associated with early lesions does not necessarily mean that an individual will develop Alzheimer's disease. This is because the brain has compensatory mechanisms that can maintain cognitive functions despite early damage. The establishment of a cohort to monitor patients at risk of developing Alzheimer's disease will enable the clinical relevance of these biomarkers in the population to be assessed. This will result in the development of a digital twin that, based on a patient's data, will predict the progression of relevant Alzheimer's disease biomarkers, using learning algorithms trained to identify patients at high risk of developing the disease.
Learning from each patient's experience to improve the management of rare diseases: application to epilepsy in children and adults
Context of the disease:
Epilepsies are chronic diseases characterized by the occurrence of seizures, which manifest as a sudden and transient disturbance in the brain's electrical activity. They appear without any identified cause or may be related to another condition. The global prevalence of epilepsy is 1% of the population.
There is a wide diversity of experiences among people with epilepsy:
- Some cases are quickly controlled through medication.
- Others, known as "drug-resistant", are not controlled through medication and may require other treatments, such as neurosurgery or neuro-stimulation.
- There are rare forms of epilepsy, including Dravet syndrome, which is often genetic in origin, begins within the first year of life, and is associated with a severe progression.
What are the challenges associated with screening for these rare diseases?
Epilepsies encompass a group of rare diseases that are clinically and biologically very different. Given the diversity of forms of epilepsy, each patient must receive a personalized diagnosis and care management plan. How can we learn to personalize care based on a small number of observations for each rare form of epilepsy?
Moreover, each observation is complex. The diagnosis and management of rare epilepsies relies on multiple medical examinations. The data are extensive, heterogeneous, and must be analyzed collectively by multidisciplinary teams. The organization and efficiency of this information exchange is a primary challenge in disseminating expertise across healthcare regions and improving medical practice as close as possible to patients.
How does the MEDITWIN project help identify and characterize each rare form of epilepsy?
The MEDITWIN project is based on the development of virtual twins built from the clinical and biological profile of each patient. They will help doctors diagnose patients with rare epilepsies by comparing patients with one another. These virtual twins will speed up diagnosis, personalize patient follow-up, and streamline the care pathway, particularly for rare forms of epilepsy such as Dravet syndrome.
In other cases, virtual twins can help identify one or more areas of the brain responsible for epileptic seizures. Epilepsy surgery will then be offered as a treatment option, with different procedures depending on the anatomical location of the epileptic zone.
Reference
Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Steinmetz, Jaimie D et al. The Lancet Neurology, Volume 23, Issue 4, 344 - 381
