Cardiology
Better understanding and treating cardiovascular or cardio-neurovascular diseases with MEDITWIN.
Toward More Precise Cardiology
The MEDITWIN project includes three pilot case studies evaluating how virtual twins in cardiology could enable earlier and more precise intervention in cardiovascular pathologies for:
Preventing cardiovascular events in people with familial hypercholesterolemia
Cardiovascular diseases are the leading cause of death worldwide, and hypercholesterolemia is a well-established risk factor.
- One in 300 people suffers from familial hypercholesterolemia, one of the most common genetic disease.
- Around 20% of people with coronary artery disease are not being treated for their cholesterol.
- Approximately 2.2 million French people (out of 45 million adults) were affected by atherosclerotic cardiovascular disease (a disease characterized by the accumulation of cholesterol plaques in the arterial walls) in 2021.
Why focus on familial hypercholesterolemia to learn how to reduce cardiovascular risk through digital twins?
Today, anyone can assess their cardiovascular risk with the help of their doctor. Indeed, certain measurable factors, such as LDL cholesterol, are indisputably linked to the risk of cardiovascular disease. However, these markers are not sufficient to closely monitor the cardiovascular health of people with familial hypercholesterolemia.
Without predictive tools, it is difficult to individualize patient monitoring and choose the most appropriate treatments and optimal doses, while reducing the side effects associated with treatments (statins). The medical teams chose to use patients with familial hypercholesterolemia as a model because this population has a much higher risk of developing cardiovascular disease. The idea is to model their medical data in order to create digital twins.
Prof. Bertrand Cariou, Director of l’Institut du Thorax (Nantes University Hospital)
"This familial hypercholesterolemia model functions as a prototype. The digital twin developed from it will subsequently be validated and adapted to other high-risk populations."
How can the MEDITWIN project help better assess and reduce cardiovascular risk?
MEDITWIN will offer the possibility of establishing a virtual twin model combining the metabolic, cardiovascular, and retinal systems to monitor how cardiovascular health evolves in people living with familial hypercholesterolemia.
The teams at Dassault Systèmes and INRIA HEKA will notably use advanced statistical approaches to analyze clinical, metabolomic, and genetic data. This approach will provide a better understanding of why some people develop cardiovascular disease or suffer heart attacks, enabling risks to be anticipated and reduced in future patients, improving their care and preventing recurrence.
Why combine multiple scales in virtual twins?
Cardiovascular events are mainly caused by damage to large arteries, such as the coronary arteries. These events become even more serious if the small vessels are also affected (which can cause a reduction in the resilience of organs to attack). The retina is the only organ where blood flow can be observed relatively easily, down to the smallest blood vessels, the capillaries. For example, optical coherence tomography (OCT) analysis can detect the after-effects of retinal "micro-infarctions" associated with cardiovascular events.
Virtual twins will be able to help better understand the links between micro- and macro-circulation in a healthy cardiovascular system in order to better understand the pathophysiological mechanisms associated with aging, hypertension, diabetes, etc. For example, a retinal circulation twin will enable highly precise, personalized analysis of the microcirculatory status, and will also allow the effect of treatment on microcirculation to be monitored, which is not currently possible. With digital twins, management will become individualized.
Dr. Matthieu Wargny (Nantes University Hospital)
"This MEDITWIN project represents real hope for patients suffering from cardiovascular diseases."
Preventing serious ventricular arrhythmias after a heart attack
What is the risk of serious ventricular arrhythmias after a heart attack?
Serious ventricular arrhythmias can occur in individuals who have suffered a myocardial infarction, whether recently or in the more distant past. These arrhythmias are disturbances in heart rhythm caused by tissue changes following the infarction.
Implanting a defibrillator in patients who have had a heart attack can help reduce this risk by detecting episodes of ventricular tachycardia and regulating the heart rhythm. However, the selection criteria used to guide defibrillator implantation only identify 30% of those at risk.
How should patients be treated after a heart attack?
It is essential to accurately identify people who are at risk of developing serious ventricular arrhythmia after a heart attack. The risk increases proportionally to the size of the infarction, which is detected by measuring the performance of the heart muscle through the ejection fraction, which will be lowered (the ejection fraction corresponds to the fraction of blood ejected by the heart into the body with each beat).
Unfortunately, this is a rudimentary assessment, and some patients are at high risk of sudden death despite a moderately impaired ejection fraction. Other criteria come into play and have yet to be identified.
How does the MEDITWIN project offer hope for preventing heart attacks?
To better identify patients at risk, virtual twins will rely on measurements from cardiac imaging (MRI) and electrocardiograms, combining them with the knowledge of medical teams.
Using non-rigid registration methods, 3D segmentation, and probabilistic predictive models, the teams from Dassault Systèmes, IHU Liryc and INRIA EPIONE will focus on developing a digital cardiac twin that can accurately assess the risk of serious ventricular arrhythmias after a heart attack and thus effectively guide prevention strategies (for example, defibrillator implantation).
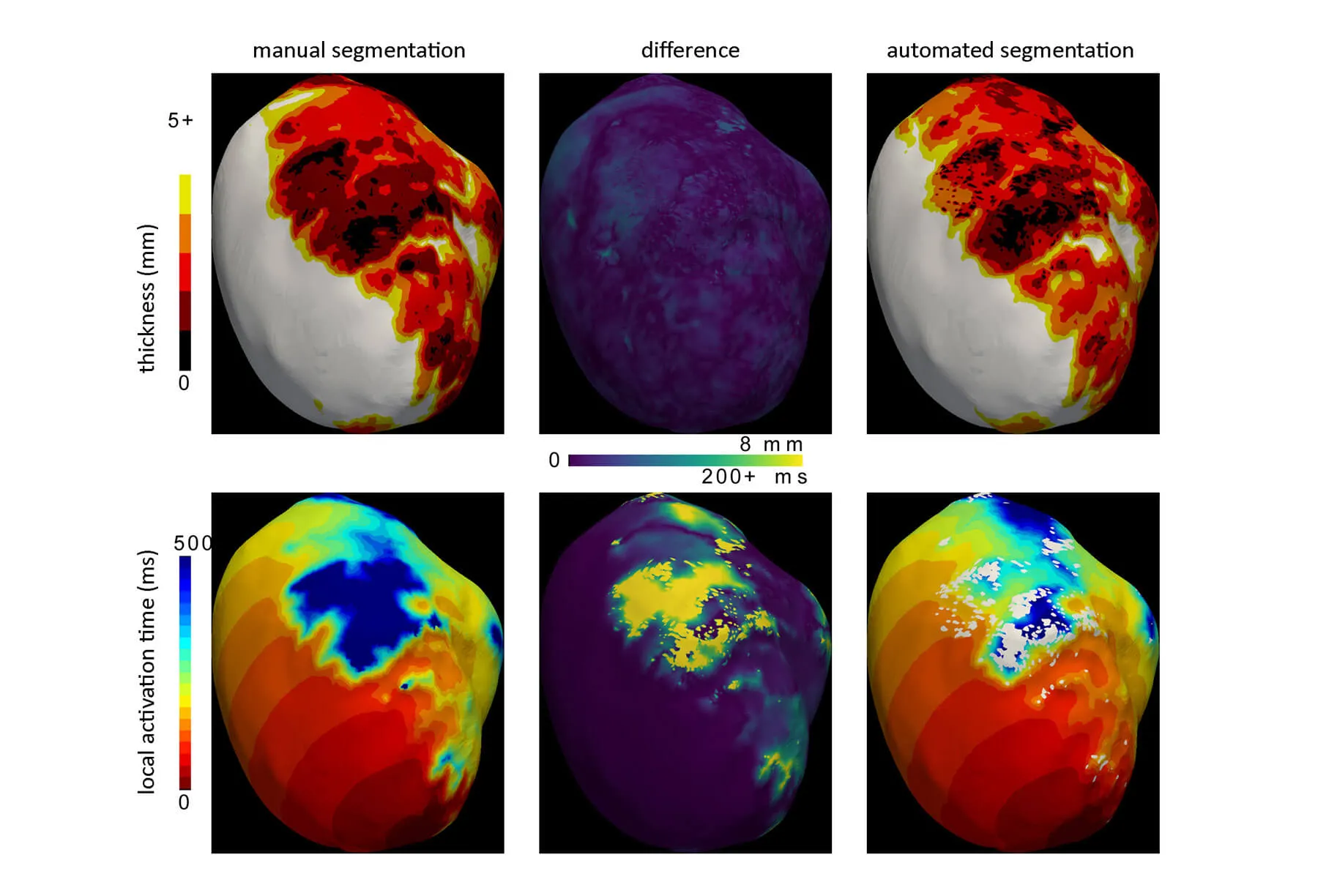
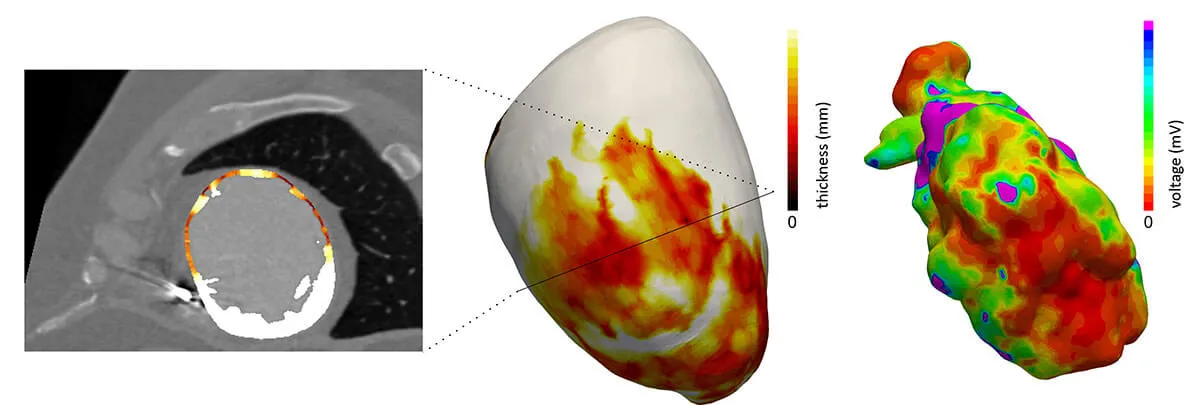
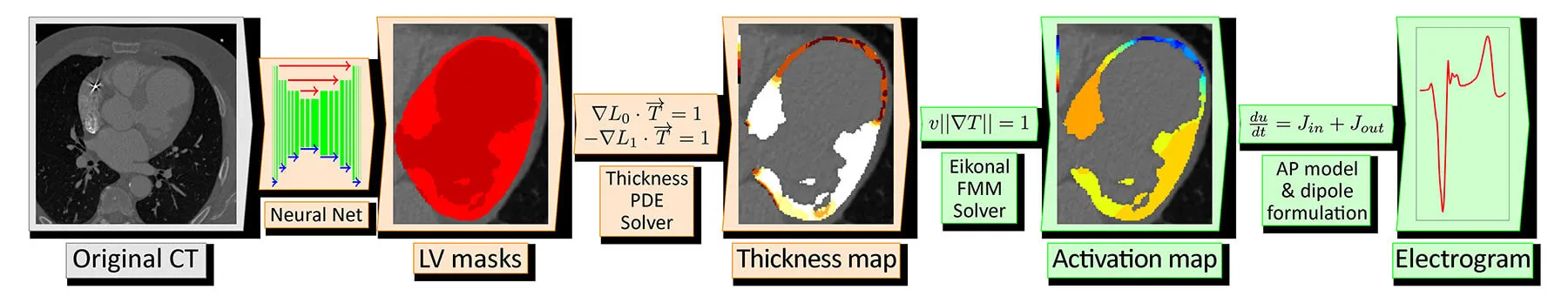
inHEART develops technologies for 3D segmentation of the heart and scar tissues from medical imaging data, including CT and MRI scans, to build a personalized digital twin of the heart.
This digital twin will be progressively enhanced by integrating additional clinical predictors, particularly derived from MRI and electrocardiogram (ECG) data, to enable a precise stratification of sudden cardiac death risk.
The overall objective of the project is to automate this critical step through the use of artificial intelligence, there by accelerating and standardizing the digital twin preparation process while ensuring a high level of reliability and reproducibility of the results.
Pediatric cardiology: improving the management of left ventricular hypoplasia in newborns
What is left ventricular hypoplasia?
Left ventricular hypoplasia is a rare disease characterized by a congenital heart defect. This condition affects the left ventricle and the major blood vessels of the heart in newborns. It is characterized by underdevelopment of the left ventricle, which no longer functions as a pump.
These newborns must undergo several heart surgeries in the first weeks and years of life to restore effective blood circulation throughout the body with a single ventricle.
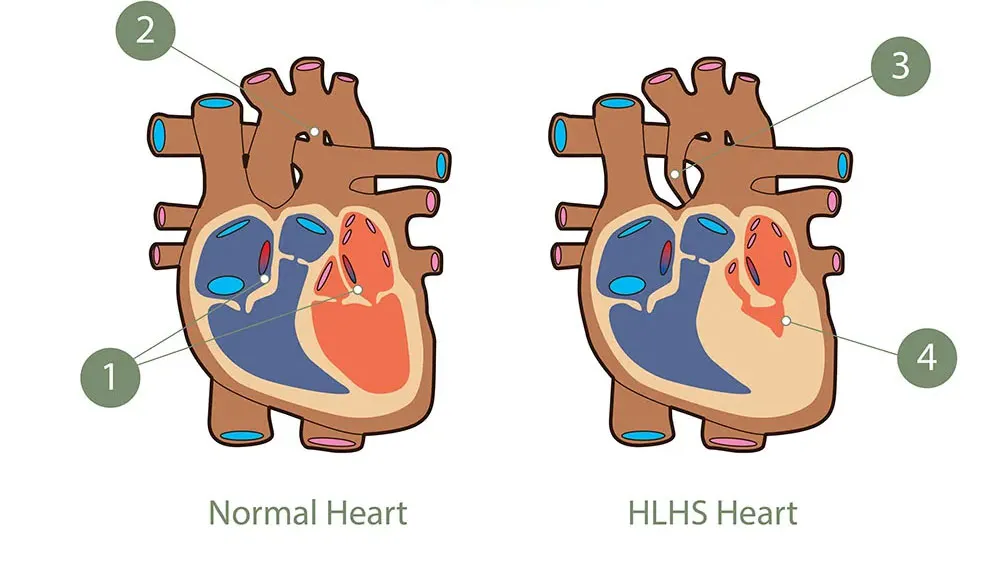
What are the challenges in treating newborns living with this condition?
Left heart hypoplasia is the most severe heart defect, impacting survival and quality of life for newborns. It requires prompt surgical intervention within the first few days after birth. Several complex interventions, potentially combining surgery and catheterization in the pulmonary arteries, will be necessary.
Newborns who have undergone surgery must receive significant medical follow-up to prevent complications and ensure better quality of life. This must be coordinated over the long term and tailored to each patient. Today, doctors often have to make critical decisions with only limited information about how the disease is progressing and how treatments are working.
What does the MEDITWIN project bring to pediatric cardiology?
With the development of personalized virtual twins of newborns' hearts, surgeons and doctors will be able to:
- Identify the anatomical areas of the cardiovascular system that need correction
- Assess the impact of various possible interventions on physiological measurements
- Take into account the evolution of the patient's condition before and during surgery to ensure continuous follow-up throughout their growth
Two Inria project teams are involved in this project:
- The COMMEDIA project team will develop numerical blood flow models based on fluid mechanics equations (Navier-Stokes equations). These finite element method (FEM) simulations will enable accurate representation of cardiac dynamics in newborns with left ventricular hypoplasia. These virtual models will provide an essential tool for preoperative planning, thereby optimizing surgical management and personalized medical follow-up for young patients.
- The SIMBIOTX project team will develop multi-scale models combining the so-called "zero-D" (compartmental models with concentrated parameters), "one-D" (flow in vascular networks), and "three-D" (detailed hemodynamic simulation by finite elements) approaches. The integration of these models within a spatio-temporal digital twin will enable the simulation of the biological and physiological evolution of children with complex cardiac conditions, such as hypoplastic left heart syndrome, thereby facilitating critical clinical decision-making.
References
Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021 Ferrari, Alize J et al. The Lancet, Volume 403, Issue 10440, 2133 - 2161