ENOVIA
Powered by the 3DEXPERIENCE platform, ENOVIA helps you deliver transformative innovations
The Generative Economy is touching all industries, bringing new ways of seeing the world, inventing, learning, producing and trading.
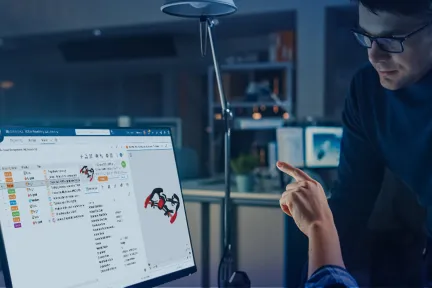
Powered by the 3DEXPERIENCE platform, ENOVIA transforms how people innovate so businesses can thrive in the modern world. By seamlessly connecting people, data and processes within a unified environment, ENOVIA empowers organizations with the knowledge and agility needed to streamline product development, optimize collaboration and enhance decision-making.
Discover how the ENOVIA portfolio helps you more efficiently build and flexibly execute your plan for success.
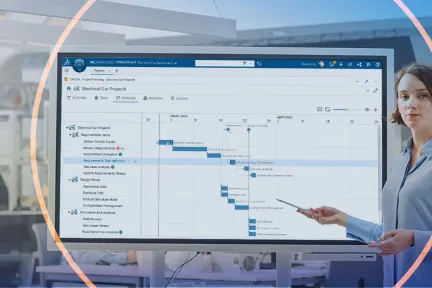
Connect people, ideas, data & processes
Gain insights from real-world data
Define a resilient products & services strategy
Turn your value network into a strategic advantage
Transform the innovation process and reduce design cycle times
ENOVIA Portfolio
Explore our Software and Solutions
Featured Topics
Discover the world of ENOVIA
Go beyond PLM, advance to enterprise transformation.
Achieve Seamless Quality with the 3DEXPERIENCE Platform
Transition to next gen PLM system
Deliver innovation in a changing world
Accelerate innovation by being agile
Seamless Collaboration for Better Outcomes
News & Offers
Customer Stories
How Leading Companies are Using our Product Lifecycle Management Solutions
AeroMobil
Redefining Personal Transportation
AeroMobil's goal is to become the world’s leader in personal transportation. The company uses the 3DEXPERIENCE platform to manage the lifecycle of its flying cars, ensure regulatory compliance and find the best design before physical prototyping.
3DEXPERIENCE boosts our ability to be creative. It accelerates our technological progress and lays the foundation for our long-term development.
From our blog
Read latest ENOVIA news, tips & tricks and other interesting topics from users and experts around the world
From the user community
Providing essential ENOVIA resources and active engagement with fellow users
ENOVIA Champions Community
The ENOVIA Champions Community is an exclusive (invitation only) space for ENOVIA Champions to meet and interact with one another and with ENOVIA's management and experts from R&D, the Product Portfolio team and the Industry Process Success team. Our champions use this space to innovate and collaborate with ENOVIA to catalyze the future and improve the way we all do business.
E-seminars
E-books, whitepapers, and other resources
Connect with ENOVIA
Learn what ENOVIA can do for you
Speak with an ENOVIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right ENOVIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering