FEops
Dassault Systèmes software has proven highly capable of accurately simulating the methods and complexities of heart-disease treatment. FEops can explore the application of their technologies to a wide range of heart-related fields, getting medical devices to market faster and helping patients everywhere.
The average heart beats some 115,200 times per day, pumping blood between the organ’s upper and lower chambers. Valves between the chambers and associated blood vessels open and close with each beat to regulate the pressure and course of blood throughout the body. It’s a beautiful system when healthy, but genetics, infections and age can all take a toll on heart valves over time.
More than 5 million Americans are diagnosed with heartvalve disease each year, according to the American Heart Association (AHA); Europe and other industrialized countries share similar statistics, with the prevalence of valvular heart disease estimated at 2.5% overall. Those numbers are anticipated to rise around the globe as populations continue to expand and age, and risk factors (diabetes, poor health habits, viruses and bacteria that can damage the heart) all continue to take a toll.
After age 65, the prevalence of heart-valve disease increases markedly, particularly aortic stenosis (calcification and narrowing), and mitral regurgitation (incomplete closure). Since the aortic valve controls the movement of oxygenated blood from the left ventricle into the aorta, the main artery leading to the rest of the body, its function is critical; without an aortic valve replacement, 50% of aortic valve disease sufferers will not survive more than an average of two years after the onset of symptoms.
An Alternative to Open-Heart Surgery
AHA’s “2018 Update” on heart disease notes that the number of elderly patients with aortic stenosis is projected to more than double by 2050 in both the United States and Europe. One study (Icelandic AGES-Reykjavik) projects a tripling of that number by 2060. Replacing diseased aortic valves has become a “routine” treatment for patients, either through open-heart surgery or transcatheter aortic valve implantation (TAVI).
TAVI, implemented first in Europe around 2008 and approved by the U.S. FDA in 2011, is being employed in greater numbers of patients every year, particularly for elderly or ill ones. It is much less invasive, as it avoids cardiopulmonary bypass, and requires less time and anesthesia than a major surgical procedure. TAVI involves inserting a catheter, usually through the femoral artery in the thigh, and then threading it up into the heart to secure a replacement valve inside the original one. The new valve is mounted on a dedicated endovascular stent.
There tend to be fewer complications overall with TAVI than with open heart procedures, but problems can still arise: stroke from calcium loosened during the procedure, restricted blood flow, leakage when the new implant doesn’t seal completely, or electrical conduction problems requiring a pacemaker. Innovative researchers have focused on mitigating these types of roadblocks to full patient recovery, making breakthrough progress with realistic simulation.
We have decades of experience using Abaqus specifically for minimally invasive cardiovascular and endovascular devices,” he says. “Abaqus can accurately simulate the complexities of the TAVI procedure and products, which allows us to be very confident in the results.
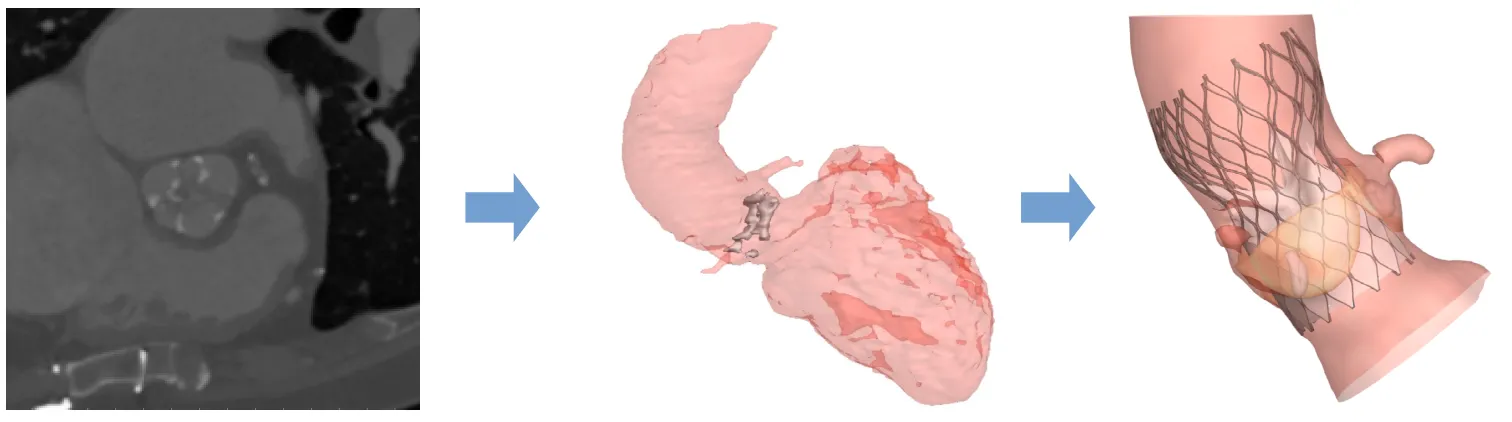
Modeling the Challenges of Valve Replacement
“The field of minimal invasive transcatheter based cardiovascular procedures is growing very fast and it underlines the need for good procedural methods,” says Matthieu De Beule, Ph.D. co-founder and CEO of Belgium-based FEops. The company is a spinoff from Ghent University, where he and co-founder, Peter Mortier, Ph.D., CTO received their doctorates. They and their team have extensive experience in advanced cardio- and endovascular device-and-procedure modeling, and collaborate with academic researchers and clinicians worldwide.
FEops HEARTguide™ technology uses advanced computational modeling to provide clinicians and valve manufacturers with insights into the interaction between valve and specific patient anatomy. The first solution of its kind, FEops HEARTguide surpasses simple anatomical measurement, generating accurate predictions on how devices will interact with each unique patient. This end-to-end solution adds value at every step in the structural heart device product life cycle, helping manufacturers accelerate time to market and bringing physicians insights that contribute to better patient outcome.
Now approved in Europe for clinical applications (CE-marked) and currently preparing FDA submission, FEops TAVIguide™ technology personalizes aortic valve implantations by combining patient scan data with predictive computer simulation. “This is the very first patient-based predictive simulation model for structural heart interventions, aimed at improving the design, planning, safety and efficacy of TAVI products and procedures,” says De Beule “and we’re proud to announce that with our most recent TAVIguide release also bicuspid patients can be analyzed.”
TAVIguide™ technology starts with pre-operative images, gathered from an individual’s CT scans. The structural geometry of the scans is extracted and used to create digital 3D anatomically correct finite element models of a patient’s aortic root. FEops employs Abaqus finite element analysis (FEA) software from Dassault Systèmes SIMULIA, along with proprietary software, to customize each model. “The technology allows the study of different virtual procedural alternatives, which helps clinicians become optimally informed before performing the real procedure,” says De Beule.
Manufacturers can also benefit from FEops technology, De Beule says. “We’re empowering manufacturers of medical devices to get their products to market faster, which ultimately helps patients everywhere.”
In the past three years we’ve come down from days to hours to run a simulation.
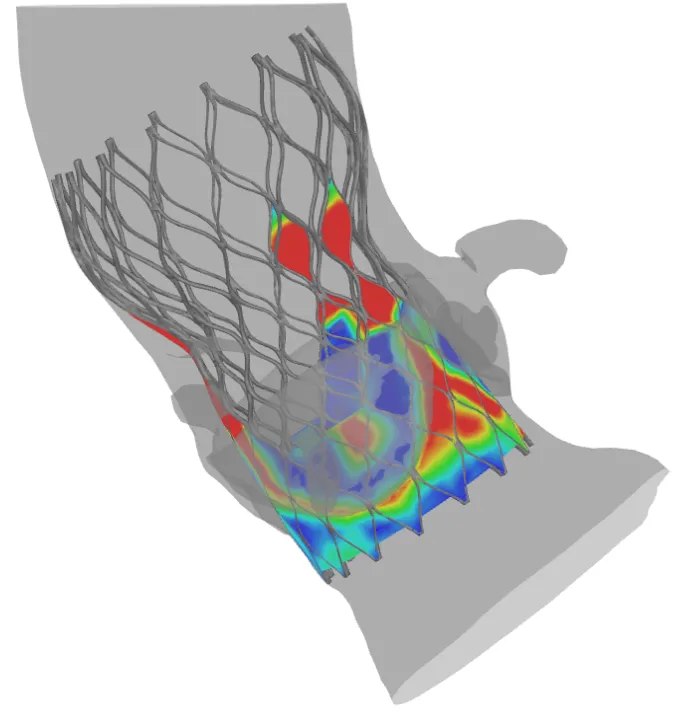
Simulation as Key to Innovation
With simulation you can gather so much more accurate information than using strictly simplified bench tests,” says De Beule. “Now you can gain a greater insight in how these devices are deployed into an individual’s anatomy, even before manufacturing one or more physical prototypes. The simulations can then predict the behavior of replacement valves during transcatheter delivery, implantation and even after new-valve function begins in a patient’s body.”
Through several collaborative retrospective studies scientific evidence was gathered with Professor Peter de Jaegere from the Erasmus Medical Center in Rotterdam, the Netherlands. In our most recent study on 112 patients, published in Circulation Interventions, we have shown that our patient-specific computer simulations are able to predict which patients are at risk for conduction abnormalities after TAVI.
Dassault Systèmes software tools have proven highly capable of replicating the complicated landscapes and methods of the TAVI procedure, De Beule points out. “We have decades of experience using Abaqus specifically for minimally invasive cardiovascular and endovascular devices,” he says. “Abaqus can accurately simulate the complexities of the TAVI procedure and devices, which allows us to be very confident in the results.”
In addition to TAVI, FEops is developing models for mitral and tricuspid valve replacement, and LAAO (left atrial appendage occlusion)—and De Beule sees their technology being applied to a wide range of other heart-related fields as well. “We are convinced that this technology and personalized approach can not only be applied to additional cardiac devices, but our simulation framework can also help medical device designers consider a variety of new cardiovascular devices in realistic and validated patient anatomies early on in the pre-clinical state of development, paving the way for virtual clinical trials,” he says.
As they’ve continued to refine their FEops HEARTguide technology, FEops is seeing advancements in software and computational resources that empower them to innovate further. “In the past three years we’ve come down from days to hours to run a simulation,” says De Beule. “I think software innovation will allow us to get close to real time in a couple more years.”
While he points out his team’s strengths in modelling strategies and workflow automation, he is quick to acknowledge the expertise of others. “We know where the strength of others lies as well, which is why we are using Dassault Systèmes software,” he says. “It’s the best there is and we want to work with the best technologies out the and link them up to our own unique expertise.”
