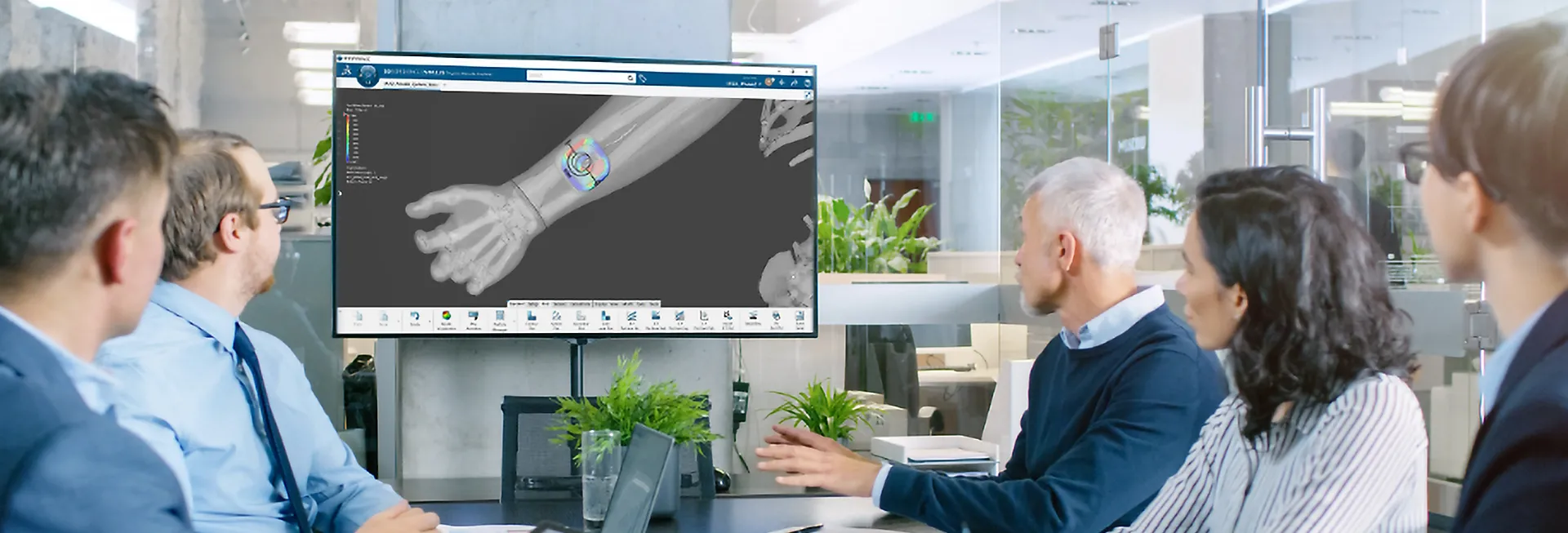
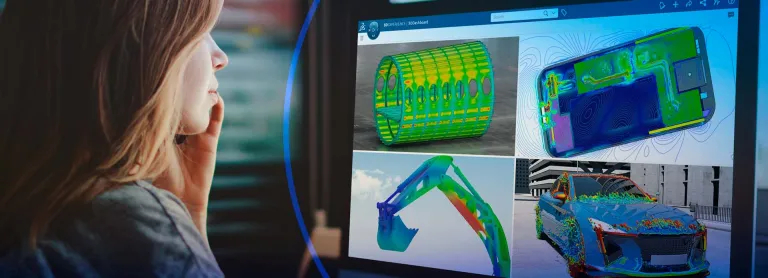
SIMULIA Life Sciences & Healthcare Solutions
SIMULIA Multiphysics simulation is driving Life Sciences innovation, helping companies to virtually test and optimize cutting-edge medical devices and treatments.
Why Does the Life Sciences and Healthcare Industry Need Modeling and Simulation?
The increasing global incidence of chronic diseases demands that novel devices, drugs, and treatments be developed to withstand stringent regulatory scrutiny while meeting tight budgets and time constraints. The expectation of measurable long-term improvement in patient outcomes also drives the need for greater personalization of devices and therapies. However, excessive reliance on physical, animal, and human testing of drugs and devices continues to delay development time and increase costs.
The SIMULIA Life Sciences & Healthcare portfolio offers best-in-class simulation solutions for structures, fluids, and electromagnetics, as well as virtual human modeling - all delivered on the 3DEXPERIENCE platform on the cloud. These solutions provide unified modeling and simulation to boost innovation, reduce development time and cost, and improve patient experience and outcome.
Key Benefits of SIMULIA Life Sciences & Healthcare Solutions
Reduce Development Costs
Reduce cost of device development by systematically adopting virtual testing in place of physical testing
Ensure Device Compliance
Meet all performance, quality, and compliance requirements by assessing device performance with multiphysics simulation and virtual human modeling
Accelerate Time to Market
Shorten time from concept through final design through Unified Modeling and Simulation (MODSIM)
Drug Delivery & Diagnostic Devices
Portable or wearable drug delivery systems and diagnostic devices have revolutionized the quality of life for their users. These devices need to be reliable, comfortable, and easy to use.
Simulation enables medical device and pharmaceutical manufacturers to test their products in silico and identify and resolve potential safety and performance issues before clinical testing.
Applications include:
- Structural integrity and impact testing
- Plastic injection molding
- Electromagnetic performance and safety
- Fluid flow and fluid-structure interaction
- Needle penetration and device-skin interaction
Stents & Cardiovascular Implants

Cardiovascular disease is one of the most significant health burdens in the developed world. Coronary and neurovascular stents are widely used to prevent arterial blockage. Additional cardiovascular implants include pacemakers, heart valve repair and replacement devices, ventricular assist devices, etc.
Simulation reduces the amount of physical testing needed and accelerates the product development cycle. It can replicate bench and in-vivo testing scenarios to identify and mitigate the risk of implant failure.
Applications include:
- Pacemaker leads
- Leadless pacemakers
- Coronary and neurovascular stents
- Transcatheter Aortic Valve Replacement (TAVR) stents
- Mitral annuloplasty rings
- Mitral clips
Orthopedic & Dental Implants
The orthopedic and dental implant market is growing rapidly, driven by an aging population motivated to maintain an active lifestyle. These factors are also driving the demand for personalized implants.
Simulation is used to evaluate implant performance throughout its entire lifespan. Personalized implants can be designed and analyzed for fit and function by integrating scan data into the simulation.
Applications include :
- Strength and stiffness
- Kinematics and dynamics
- Durability, wear, and lifespan
- Flow analysis in porous implants
- Manufacturing and 3D printing process optimization
- MRI safety

Pharmaceuticals & Biotech
Developing a new pharmaceutical or biotech therapy is highly expensive and time-consuming. Modeling and simulation helps to reduce risk, lower development costs, and speed therapeutics to market. Simulation is used for a broad range of pharma and biotech applications, such as improving the drug product and manufacturing processes, optimizing drug delivery device performance, evaluating packaging usability and integrity, and ensuring safe environments by analyzing and improving airflows in manufacturing facilities.
Applications include:
- Tablet compaction
- Drug delivery and diagnostic devices
- Packaging for optimal pharmaceutical storage, transportation, and usability
- Manufacturing plant air quality
- Bioreactors and mixing tanks
Hospital Air Quality Simulation
Airborne transmitted infections are invisible but everywhere and significantly impact the quality of care provided by hospitals. They affect patient outcomes, increase healthcare costs, prolong hospital stays and even lead to mortality.
Hospital HVAC systems can be modeled and simulated to optimize ventilation and air quality, improve the safety of patients and staff, maintain patient comfort, and lower energy costs in all climate conditions.
Applications include:
- Airborne disease propagation
- Surface contamination
- Air quality and indoor pollution
- HVAC optimization
- Lab biosecurity and clean room design
Bioelectromagnetics
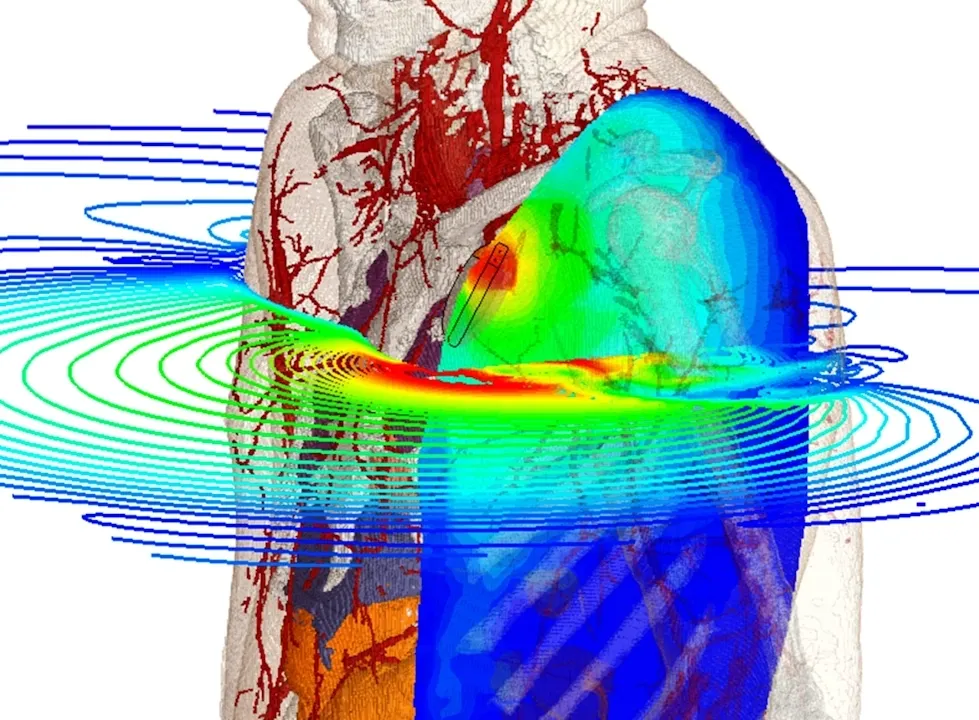
Bioelectromagnetics (BioEM) covers the interaction of electromagnetic (EM) fields with the human body. It is a crucial topic for a wide range of medical devices for imaging and treatment. The human body is a complex structure made of materials with a wide range of frequency-dependent EM and thermal properties. To approach these complexities we need specialized modeling and simulation methods. BioEM simulation models the propagation of waves through the body, and the interaction of waves with tissues – whether a required therapeutic effect or an unwanted side effect. Device engineers can use the simulation results to understand exactly how energy is absorbed by the body and validate designs and treatment protocols.
Applications include:
- MRI design
- Diathermy and hyperthermy
- RF and Microwave imaging
- Wearable and implant electronics
- Implant safety
Virtual Human Modeling & Simulation
To understand the safety and efficacy of a new medical device, it is imperative to analyze its interaction with the human body.
Virtual Human Modeling & Simulation, from Dassault Systemes, enables medical device companies to assess the safety and efficacy of their products within a realistic virtual human environment. It offers the ability to create detailed models of human organs and systems, such as the heart and musculoskeletal system. It also provides the capability to modify these models to create or patient-specific twins or a large set of virtual patients which can be used to improve medical device safety and effectiveness.
Applications include:
- Tissue modeling (skin, bone, etc.)
- Human organ models (brain, lungs, eye, etc.)
- Living Heart Model
- Musculoskeletal systems modeling

SIMULIA Customers in Life Sciences and Healthcare
SIMULIA Industry Solutions for Life Sciences & Healthcare
The need for more precise, sustainable patient care, have made the Life Sciences and Healthcare industry ripe for transformation by shifting the focus to deliver superior, patient-centric, outcome-driven experiences and creating the processes to support them.
Start Your Journey
The world of Life Sciences & Healthcare Solutions is changing. Discover how to stay a step ahead with SIMULIA.
FAQs about Life Sciences & Healthcare Solutions
Also Discover
Learn What SIMULIA Can Do for You
Speak with a SIMULIA expert to learn how our solutions enable seamless collaboration and sustainable innovation at organizations of every size.
Get Started
Courses and classes are available for students, academia, professionals and companies. Find the right SIMULIA training for you.
Get Help
Find information on software & hardware certification, software downloads, user documentation, support contact and services offering